Carboxyl Group Definition
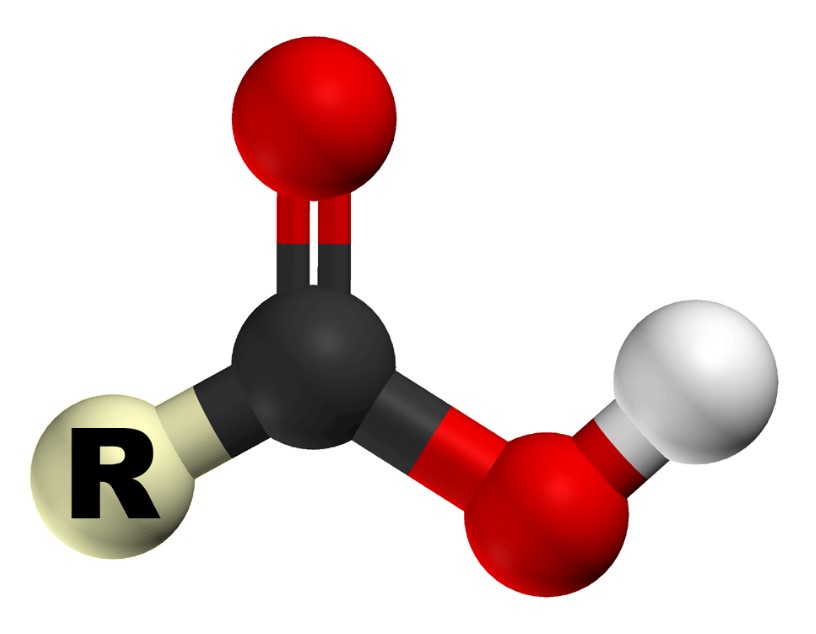
The carboxyl group is one of many functional groups that attach to larger molecules to give them their properties. Carboxylic acids are organic molecules with a carboxyl group that serve a variety of functions. Carbon forms the carboxyl group, which is bonded to oxygen and hydroxyl groups. A hydrogen bonded to an oxygen is called an hydroxyl group.
Hydrogen is attracted to the electronegative double-bonded oxygen. The hydroxyl group does the opposite, and would gladly give up a hydrogen for another carbon bond. Because carboxyl groups are polar, they can form hydrogen bonds and participate in a variety of other important reactions.
In the above diagram, “R” can be any number of carbon-containing molecules or even a single hydrogen atom. Protein synthesis is a good example of carboxyl groups. There are carboxyl groups and amino groups in every amino acid.
A peptide bond is formed between these groups, allowing amino acids to be chained together in long sequences. There are a number of biological functions performed by carboxyl groups and they are attached to a wide variety of molecules.
Related Biology Terms
- Functional Group – A section of a molecule with specific chemical properties.
- Hydroxyl Group – Oxygen bonded to hydrogen, which can exist free in solution or attached to molecules.
- Carboxylic Acid – An organic molecule that contains a carboxyl group that can donate a proton to a number of reactions.